Physics Heroes & Heroines, Part IV
We’ve only gotten through February, and already 2021 has seen some significant scientific achievements, breakthroughs, and new research results—from the Mars landing of NASA’s Perseverance rover to the continuing proliferation of effective COVID-19 vaccines.
In recognition of all the ongoing, vital scientific research and technology innovation happening around the globe, Radiant continues our series on Physics Heroes & Heroines (Read Part I, Part II, and Part III), spotlighting just a few of the physicists we admire:
Shuji Nakamura (1954 – )

Many modern technology devices would not be possible without Nakamura, whose invention of the blue LED (light emitting diode) changed lighting and displays forever. Born in Japan, Nakamura studied electrical engineering, then worked at the chemical and electronics firm Nichia in the 1990s. While there, he conducted the research which would later win him the 2014 Nobel Prize in Physics jointly with Isamu Akasaki and Hiroshi Amano.
Green and red LEDs had been developed decades earlier in the 1950s and 1960s, but producing a blue version proved to be far more difficult. To make an LED requires two different semiconductor layers, one with extra electrons (n-type) and one with open “holes” to receive those electrons (p-type). When voltage is applied, electrons can move from the n layer to the p layer, releasing energy; with enough energy they produce visible light. Red, yellow, and green LEDs can be created by using different materials for the semiconductors and adding chemical impurities (“doping”).1
But making blue LEDs requires more energy, which places extra demands on the semiconductor material layers. Nakamura and his collaborators worked with semiconductors using difficult-to-handle gallium nitride (GaN) until they could produce high-quality indium gallium nitride crystals (InGaN) that enabled the creation of efficient blue LEDs. Their work made it possible to create white light sources – either by mixing the outputs of blue, red, and green LEDs, or by exciting a phosphor material with a blue LED to generate green and red light.2
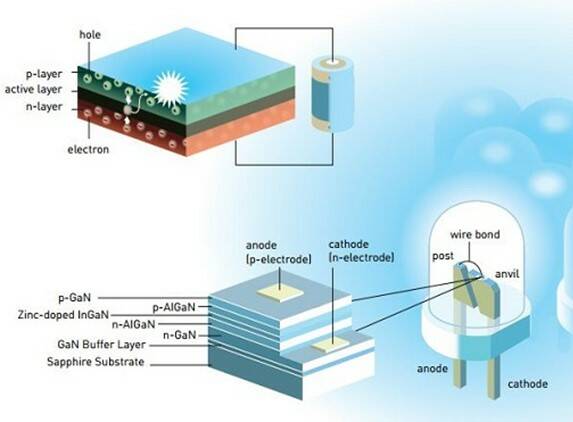
LEDs consist of semiconductor n-type and p-type layers (top image). A modern blue LED uses Gallium Nitride in combination with other elements (bottom image) to generate various colors of light. (Image: © Johan Jarnestad/Royal Swedish Academy of Sciences)
After receiving a PhD from the Tokushima University in 1994, Nakamura joined the faculty of the University of California, Santa Barbara in 1999, where he has continued his work on GaN-thin film technology for the developments of high efficient Nitride-based LEDs and laser diodes.
As NOVA's Don Lincoln explains: “LEDs can now emit far more light using far less power than incandescent and fluorescent bulbs. For instance, an incandescent light bulb can emit about 16 lumens per watt of electrical power, and a fluorescent light manages about 70 lumens per watt. In comparison, a modern white LED can emit 300 lumens per watt, meaning that it consumes only about 5% of the power of an incandescent.”3
Chien-Shiung Wu (1912 – 1997)
Wu was born in China, where her father ensured she received schooling—uncommon for girls at that time. She studied physics at National Central University in Nanking, China (now known as Nanjing University) with a professor who had worked with Marie Curie. In 1936 she traveled to the US, earning her PhD from the University of California Berkeley in 1940. In 1944, she joined the Manhattan Project at the Substitute Alloy Materials (SAM) Lab at Columbia University where she made significant contributions to both theoretical physics and research.
Notably, she was the first scientist to confirm Enrico Fermi’s theory of beta decay, she helped develop the process to split uranium into its radioactive isotope, uranium-238, and to improve Geiger counters, which measure nuclear radiation levels.
After World War II, she became a professor at Columbia University where she continued conducting research.
Expanding on her work with beta decay, she went on to prove that radioactive particles do not always behave the same way: the law of conservation of parity does not hold true during beta decay, showing that parity is not conserved under weak nuclear interactions.” The 1957 Nobel Prize in Physics was awarded to Tsung Dao Lee and Chen Ning Yang for this discovery, but it was Wu’s work that provided the foundation.4
Wu also contributed to medical research, helping physicians understand the molecular changes in blood cells related to sickle cell anemia. She was the first woman to serve as president of the American Physical Society, was awarded the National Medal of Science, the Comstock Prize, and was the inaugural recipient of the Wolf Prize in Physics. Her 1965 book Beta Decay is still a standard reference for nuclear physicists.

Chien-Shiung Wu, 1958 (Image: Acc. 90-105 - Science Service, Records, 1920s-1970s, Smithsonian Institution Archives)
Johannes Kepler (1571 – 1630)

Johannes Kepler was a German astronomer, during a time when astronomy and other sciences were part of a matrix of theological, astrological, and physical ideas. He was born in the small town of Weil der Stadt and won a ducal scholarship to study at the University of Tubingen with one of the leading mathematicians and astronomers of the day, Michael Maestlin.
Turning from his original intention to become a theologian, Kepler was captivated by the study of astronomy. He built on the earlier work of Copernicus who had proposed a heliocentric model of the universe—that the sun, not the earth, was at the center with planets circling it.
Kepler published several influential work throughout his lifetime, such as Harmonices Mundi (Harmony of the World), published in 1619. In his work he suggested that the planets have an elliptical orbit around the sun, and he outlined several principles of planetary motion—now called Kepler’s Laws—later confirmed by Sir Isaac Newton.
Kepler’s first law states that the planets move in ellipses, with the sun at one of the foci of the ellipse. The second law holds that a line between the sun and the planet sweeps equal areas in equal times, in other words, the planet’s speed increases when it’s closer to the sun and slows as it recedes. The third law established a mathematical relationship between the distance of the planets from the sun and their orbital periods. These laws enable us to understand the motion of any smaller object (a planet, satellite, space station, etc.) around a larger body (the sun, the earth, etc.).5
Kepler also made significant contributions to the science of optics, providing a novel and correct description of how human vision occurs and a new telescope design that took advantage of the behavior of light.
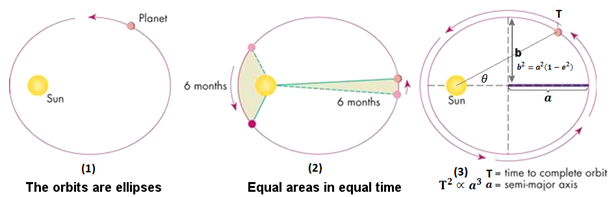
Kepler’s three laws of planetary motion describe how planets move around the sun in elliptical orbits (1), how they move faster as they get closer to the sun (2), and the mathematical relationship between their orbital period and distance from the sun (3). (Image: Source)
Elmer Imes (1883 – 1941)
Born to a missionary family in Memphis Tennessee, Imes studied at Fisk University in Nashville, a predominantly African American school, to earn his undergraduate and master’s degrees in physics, while also working as a physics and math teacher. Because Fisk didn’t offer higher degrees, Imes transferred to the University of Michigan (UM), becoming only the second African American ever to receive a PhD and the first to be able to pursue a career in research.
His work at UM involved infrared spectroscopy of three diatomic gases: hydrogen bromide (HBr), hydrogen chloride (HCl), and hydrogen fluoride (HF), and he designed and built a series of IR spectrometers in the process. In one of the earliest applications of high-resolution infrared spectroscopy, he provided the first detailed spectra of simple molecules. His research opened up the field of studying molecular structure through infrared spectroscopy and presented the first accurate measurement of the distance between atoms in molecules.
His work also provided a verification of quantum theory. “Up until the work of Imes, there was doubt about the universal applicability of the quantum theory to radiation in all parts of the electromagnetic spectrum. Imes’s work formed a turning point in the scientific thinking, making it clear that quantum theory was not just a novelty, useful in limiting fields of physics, but of widespread and general application.”6
Despite his achievements, Imes was only offered faculty positions at historically Black universities, which didn’t have PhD programs, so he spent the next decade working in the private sector as a research physicist for several engineering firms, resulting in four patents for instruments to measure magnetic and electric properties of various materials. Living in New York, he was part of the vibrant 1920s Harlem Renaissance scene that included luminaries such as Langston Hughes and W.E.B. DuBois.
After a decade, he became frustrated with racial barriers to corporate advancement and returned to academia in 1930 as the Chairman of the Department of Physics at Fisk University. He built up the department curriculum and rigor, and was active in training students, a number of whom went on to receive PhD’s from UM and other top-ranked universities.7 He returned to New York in 1939, performing research into magnetic materials at New York University until his death in 1941.

Physicist Elmer Imes and his ever-present pipe. (Image: Fisk University, John Hope and Aurelia E. Franklin Library, Special Collections, Source)
CITATIONS
- Lincoln, D., “How Blue LEDs Work, and Why They Deserve the Physics Nobel”, NOVA, October 9, 2014
- Stoye, E., “Inventors of blue LED win physics Nobel”, Chemistry World, October 6, 2014
- Lincoln, D., “How Blue LEDs Work, and Why They Deserve the Physics Nobel”, NOVA, October 9, 2014
- Chien-Shiung Wu, Atomic Heritage Foundation, https://www.atomicheritage.org/profile/chien-shiung-wu. (Retrieved February 24, 2021).
- Westman, R., “Johannes Kepler”, Britannica (Retrieved February 24, 2021)
- Dubay, K., “A Scholar and a Trailblazer”, Rackham Graduate School, University of Michigan, December 20, 2018.
- “This Month in Physics History: November 1919: Elmer Imes Publishes Work on Infrared Spectroscopy”, American Physical Society (APS) News, Vol. 17:10, November 2008
Join Mailing List
Stay up to date on our latest products, blog content, and events.
Join our Mailing List
